IONIC EQUILIBRIUM IN SOLUTION
`=>` Under the effect of change of concentration on the direction of equilibrium, you have incidently come across with the following equilibrium which involves ions:
`color{red}(Fe^(3+) (aq) +SCN^(-) (aq) ⇌ [Fe(SCN)]^(2+) (aq))`
There are numerous equilibria that involve ions only.
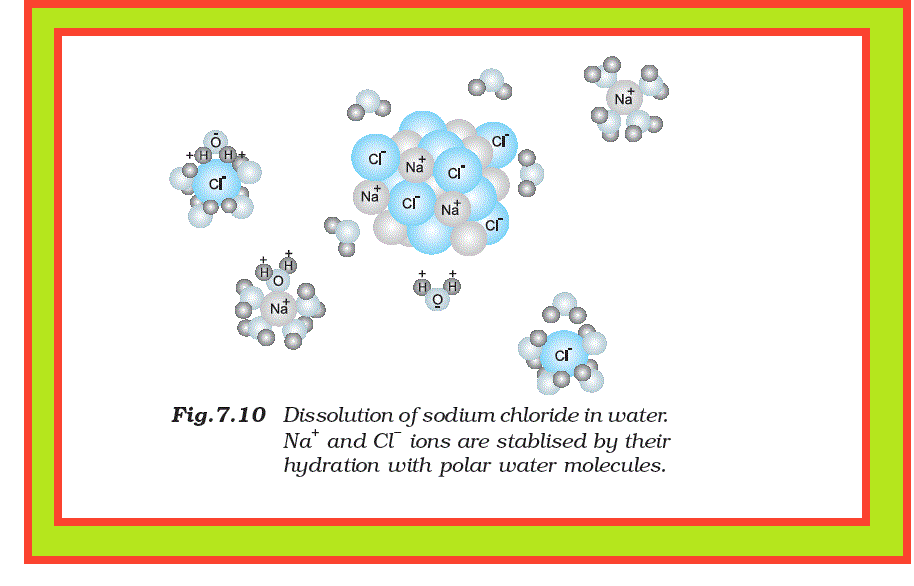
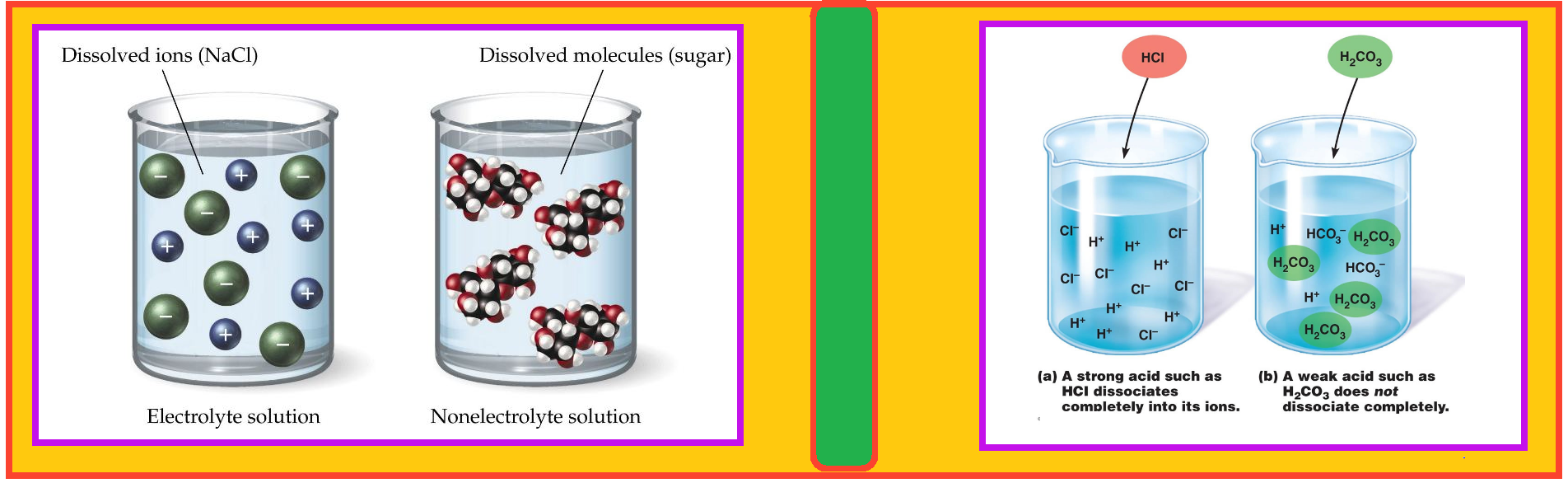
`=>` It is well known that the aqueous solution of sugar does not conduct electricity. However, when common salt (sodium chloride) is added to water it conducts electricity. Also, the conductance of electricity increases with an increase in concentration of common salt.
`=>` Michael Faraday classified the substances into two categories based on their ability to conduct electricity.
•One category of substances conduct electricity in their aqueous solutions and are called electrolytes.
•while the other do not and are thus, referred to as nonelectrolytes.
`=>` Faraday further classified electrolytes into strong and weak electrolytes.
• Strong electrolytes on dissolution in water are ionized almost completely.
• The weak electrolytes are only partially dissociated.
For example, an aqueous solution of sodium chloride is comprised entirely of sodium ions and chloride ions(there is almost `100%` ionization in case of sodium chloride ), while that of acetic acid mainly contains unionized acetic acid molecules and only some acetate ions and hydronium ions( less than `5%` ionization of acetic acid which is a weak electrolyte). It should be noted that in weak electrolytes, equilibrium is established between ions and the unionized molecules.
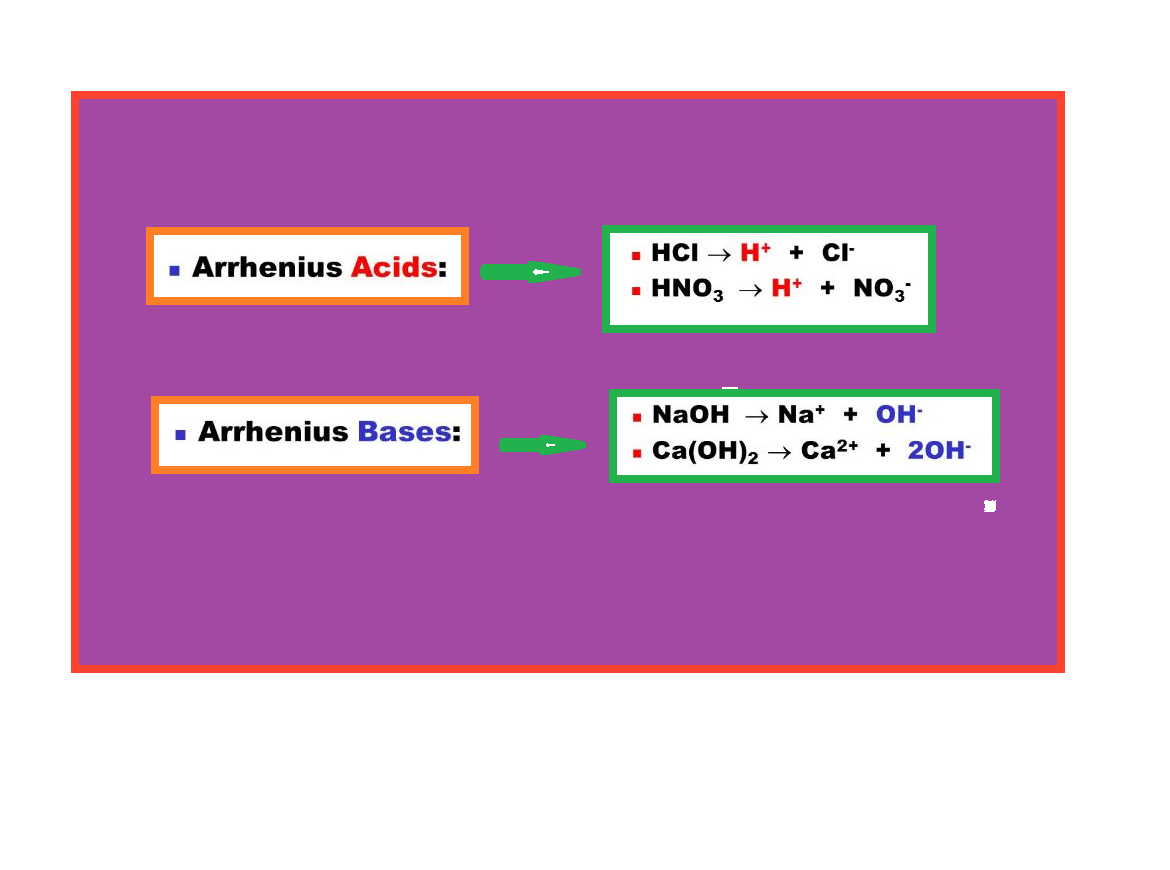
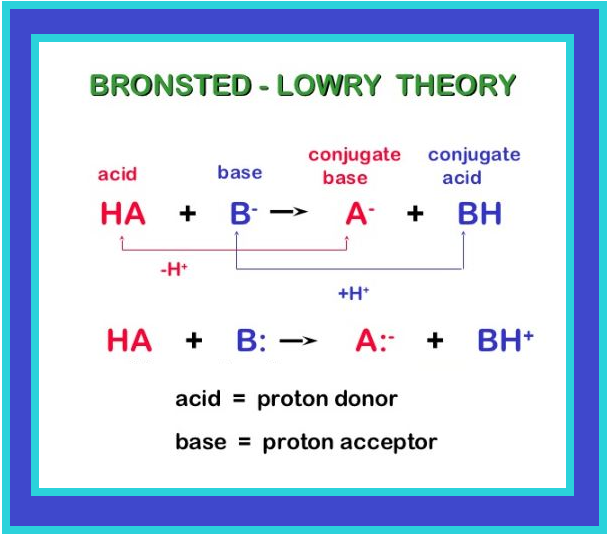
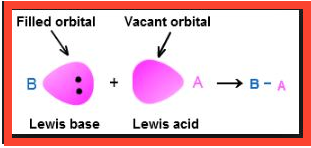
This type of equilibrium involving ions in aqueous solution is called ionic equilibrium. Acids, bases and salts come under the category of electrolytes and may act as either strong or weak electrolytes.
`color{red}(Fe^(3+) (aq) +SCN^(-) (aq) ⇌ [Fe(SCN)]^(2+) (aq))`
There are numerous equilibria that involve ions only.
`=>` It is well known that the aqueous solution of sugar does not conduct electricity. However, when common salt (sodium chloride) is added to water it conducts electricity. Also, the conductance of electricity increases with an increase in concentration of common salt.
`=>` Michael Faraday classified the substances into two categories based on their ability to conduct electricity.
•One category of substances conduct electricity in their aqueous solutions and are called electrolytes.
•while the other do not and are thus, referred to as nonelectrolytes.
`=>` Faraday further classified electrolytes into strong and weak electrolytes.
• Strong electrolytes on dissolution in water are ionized almost completely.
• The weak electrolytes are only partially dissociated.
For example, an aqueous solution of sodium chloride is comprised entirely of sodium ions and chloride ions(there is almost `100%` ionization in case of sodium chloride ), while that of acetic acid mainly contains unionized acetic acid molecules and only some acetate ions and hydronium ions( less than `5%` ionization of acetic acid which is a weak electrolyte). It should be noted that in weak electrolytes, equilibrium is established between ions and the unionized molecules.
This type of equilibrium involving ions in aqueous solution is called ionic equilibrium. Acids, bases and salts come under the category of electrolytes and may act as either strong or weak electrolytes.